- Nature Biotechnology07June 2011
Article tools
T cells rely on JAK kinases for cytokine signaling through type Iand type II cytokine receptors, leading to JAK/STAT phosphorylationand nuclear translocation and gene transcription. Companies aretargeting JAKs to treat rheumatoid arthritis and other autoimmunediseases. Incomplete, sporadic Jak inhibition may create atherapeutic window and avoid immunosuppression.
Late-stage trial results for Pfizer's first-in-class small-moleculeJanus kinase (JAK) inhibitor tofacitinib in rheumatoid arthritishave raised expectations for regulatory approval as early as nextyear. All five pivotal phase 3 trials met their primary endpoints,the New York–based pharma announced at the end of April. Ifapproved, the oral JAK inhibitor is set to rival biologicals suchas Abbott's Humira (adalimumab) that dominate the roughly $13billion rheumatoid arthritis global market for biologicals.Pfizer's tofacitinib “could be a multibillion [dollar] product forPfizer,” says John Boris, a pharmaceutical analyst for CitigroupGlobal Markets in New York. Its ultimate fate in the marketplacedepends on long-term safety data, although as newer and morespecific JAK inhibitors move towards market, Pfizer's current poleposition may soon be challenged.
JAK inhibition is a novel approach for treating a variety ofautoimmune and inflammatory diseases. JAK inhibitors interruptsignaling downstream of a multiplicity of cytokines, rather thanblocking one cytokine at a time, as is the case for otherbiological rheumatoid arthritis treatments such as tumor necrosisfactor (TNF) and intlerleukin-6 (IL-6) blockers. “Inhibiting manycytokines—a little bit—in a disease like rheumatoid arthritis, inwhich many cytokines are found in the synovial tissue and many areimplicated in the disease process, might be a useful approach,”says David Fox, a rheumatologist at the University of Michigan inAnn Arbor, who was not involved in tofacitinib trials. “You don'tthrow all your eggs in one basket as you do with a biologic againstone particular cytokine.”
The Pfizer molecule is indeed effective in rheumatoid arthritis. Inone phase 3 trial, reported at the 2010 annual meeting of theAmerican College of Rheumatology (ACR), tofacitinib monotherapyachieved a 20% or greater reduction in the number of tender andswollen joints and other parameters (ACR20 criteria) in 66% ofparticipating individuals, including ACR50 and ACR70 responses in37% and 20% of individuals, respectively. Although trial designsand populations were different, rendering comparisons problematic,these numbers are an improvement over those obtained for Remicade(infliximab), from Centocor, a division of Johnson& Johnson, in its 1999 pivotal rheumatoid arthritistrial. Remicade earned$5.8billion for Centocor in 2009. Tofacitinib, like otherdisease-modifying rheumatoid arthritis drugs, has also demonstratedthe ability to slow structural joint damage.
Pfizer expects to file for tofacitinib registration in the US andEurope by the end of 2011. In the US, a standard 10-month Food andDrug Administration review is likely, said Boris. The product couldthus launch by the end of 2012, and Citigroupforecasts$800million in worldwide sales in 2015. In general, “we don't expectpatients who are currently being treated [with biologics] to betaken off therapy and transferred to the oral,” Boris said. Foxagrees that adoption will be gradual. “A lot of rheumatologistswill probably be cautious and still use what they have experiencewith, which is TNF inhibitors and some of the newer biologics,” hesays, “and then reserve the kinase inhibitors for patients who havesome sort of difficulty or inadequate response to the biologic.”Acceptance would grow over time if tofacitinib proves to be “robustand not too dangerous in clinical practice,” he added.
Long-term safety is a major issue, because JAK signaling isfundamental to biology, and JAK inhibitors like tofacitinib tread afine line between therapeutic down-modulation of autoimmunity andoutright immunosuppression. (Tofacitinib was first conceived as animmunosuppressant for use in organ transplantation.) The fourmembers of the JAK family—JAKs 1, 2 and 3, and TYK2—play a crucialrole in immunity by enabling cytokines, secreted proteins that helporchestrate the immune response, to signal through their receptors.Roughly 60 cytokines signal through type I and type II cytokinereceptors, which have no catalytic kinase activity of their own.These receptors instead rely on JAK kinases for receptorphosphorylation, which creates a docking site for STATtranscription factors. Knocking out Jak1 and Jak2 in mice resultsin perinatal and embryonic lethality, respectively, and Jak3knockouts have severe combined immunodeficiency.
At therapeutic doses, tofacitinib avoids immunosuppression probablybecause it only partially and transiently blocks its targets. “Atthe doses being used you inhibit a lot of cytokines, but you don'tinhibit them completely,” says John O'Shea, an immunologist at theNational Institutes of Health in Bethesda, Maryland. Side effectsseen so far in the tofacitinib trials include infections (mostlymild to moderate) and minor decreases in neutrophils. Hemoglobingoes down, presumably because JAK2 is important for red blood cellformation, and low-density lipoprotein and high-density lipoproteinboth go up. Overall, “I don't think [tofacitinib] looks any moretoxic than what you get with injectable therapy,” says Boris.
A particular concern for JAK inhibitors is cardiac events becausethere is evidence that JAKs play a protective role incardiomyocytes (heart muscle cells). In April, an abstractpublished in advance of the European League Against Rheumatismmeeting reported four deaths in one of the studies, but Pfizer saidthat only one death, from respiratory failure, was reported by theinvestigator as drug related. Not all phase 3 tofacitinib data hadbeen released as of early May, but the drug doesn't appear to causeheart attacks and strokes. “I don't think there's anythingsignificant... that would lead us to believe that there is anyimpact on cardiac muscle or myocytes,” says Boris. “But again westill have to see the full data set come out.”
Of other JAK inhibitors in development, the furthest along isINCB28050 from Incyte in Wilmington, Delaware, having completedphase 2a in rheumatoid arthritis (Table1). The drug has been licensed to Eli Lilly of Indianapolis forinflammatory and autoimmune indications, and so far its efficacyclosely mirrors that of tofacitinib. The main difference is thatwhereas tofacitinib hits JAKs 1, 2 and 3, INCB28050 is specific forJAKs 1 and 2 while sparing 3. “JAK3 inhibition is extra baggage,”says Incyte president and CEO Paul Friedman, explaining that asJAK3 is not involved in IL-6 signaling, which is deemed critical,it made little sense to block it. “The reason Pfizer's [compound]does well clinically is certainly due to JAK1 and JAK2effects.”
Table 1: Drugs targeting JAK kinases in rheumatoid arthritisFull tableVertex Pharmaceuticals in Cambridge, Massachusetts, and Galapagos,in Mechelen, Belgium, chose even tighter specificity for their JAKinhibitors. Vertex's molecule VX-509, currently in phase 1, isspecific for JAK3, largely because JAK3 expression is mainlylimited to lymphoid tissues. Galapagos, in contrast, chose toinhibit only JAK1, thus retaining the ability to block signalingdownstream of IL-6 while avoiding the anemia that's caused by JAK2inhibition and any immunosuppressive effects that may come withblocking JAK3. That could create a wider therapeutic window. JAK1specificity “allows us to move up in dosing to a range where wehope we can extend efficacy without hitting JAK2-related sideeffects,” says Gerben van't Klooster, development project leaderfor Galapagos's compound, GLPG0634. The compound so far has beentested only in healthy volunteers, and will enter phase 2 inrheumatoid arthritis in the second quarter of 2011.
But Paul Changelian, former director of inflammation biology atPfizer, believes Pfizer's pan-JAK approach is correct and thatinhibition of all three JAKs contributes to tofacitinib's efficacy.He points out that IL-15, which boosts proliferation of naturalkiller cells, signals partly through JAK3. And the IL-23 receptorsignals partly through JAK2, controlling differentiation of Th17helper T cells, considered key mediators of inflammation. Soinhibiting both JAK3 and JAK2, as well as JAK1, may beimportant.
But Changelian concedes that tofacitinib may eventually be betteredby a different JAK inhibitor. At least 16 companies have filedpatent applications on JAKs. “People are convinced that there'sstill a better molecule to be had there,” he says. “And they may beright.”Few of these effects were known when Pfizer began itsJAK inhibitor program in the mid-1990s. Company chemists designedtofacitinib, first synthesized in 2000, to specifically inhibitJAK3. But the results from solid phase kinase-specificity assaysthat indicated JAK3-only inhibition later proved incorrect.Cellular assays also were misleading because signaling downstreamof most cytokine receptors requires the association of twodifferent JAKs, and it's hard to know whether drug potency is theresult of inhibiting just one, or both. As a result, Pfizeraccidentally made tofacitinib a pan-JAK inhibitor—a fortuitousoutcome, says Changelian, because the drug somehow achieves theright balance of inhibition across the JAKs without hitting non-JAKkinases and causing toxicity. “It's remarkably clean across thekinome,” Changelian says. “That really is what's important.”
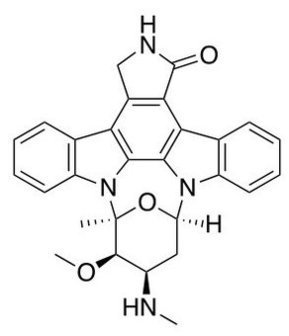
Table1: Drugs targeting JAK kinases in rheumatoid arthritis
| Company | Agent | Target | Stage |
|---|---|---|---|
| Pfizer | Tofacitinib (CP-690,550) | JAK3, JAK1, JAK2 | Phase 3 (six trials) |
| Incyte/Eli Lilly | LY3009104 (INCB28050) | JAK1, JAK2 | Phase 2b |
| Vertex Pharmaceuticals | VX-509 | JAK3 | Phase 2 |
| Galapagos | GLPG0634 | JAK1 | Phase 1 complete |
 爱华网
爱华网